HALO Diagnostics' Prostate Cancer Program
The HALO Dx Approach
At HALO Diagnostics, we use multiparametric magnetic resonance imaging (mpMRI)—combined with PSA testing—for the timely and accurate diagnosis of prostate cancer.
The American Urologic Association (AUA) now recommends mpMRI for Diagnosis of prostate cancer and prior to any biopsy. mpMRI can not only detect prostate cancer more accurately than other tests, it can differentiate between aggressive and slow growing cancers. This can help you avoid an unnecessary biopsy.
If a biopsy is needed, mpMRI can be used to more precisely guide the biopsy, ensuring that sampling is not “blind”—randomly taken from across the gland—which may completely miss the disease.
mpMRI: A clinically proven innovation
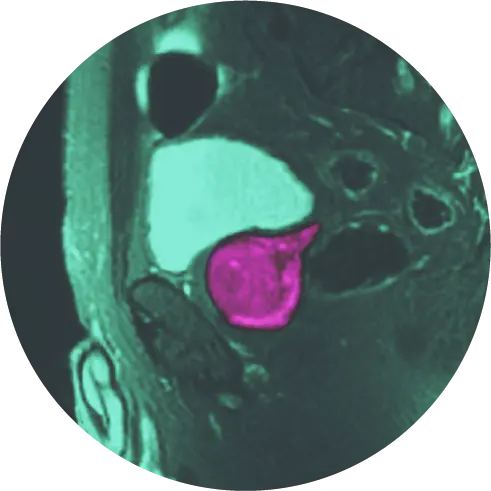
Multiparametric magnetic resonance imaging (mpMRI) is a powerful tool used to identify areas of the prostate that might be suspicious for clinically significant cancer. mpMRI is based on well-established MRI scanning technology and provides a detailed image of the prostate.
mpMRI provides a more holistic view of the prostate compared with more traditional approaches. While only a biopsy can diagnose cancer, mpMRI can assist with the characterization of areas within the gland that may warrant biopsy. Given the utility of mpMRI, the AUA recommends mpMRI for men with high PSA and BEFORE biopsy in their most recent policy statement.
mpMRI uses three different types of images to see inside the prostate gland. The first are T1- and T2-weighted imaging which show the anatomy of the gland. Second is diffusion- weighted imaging (DWI) which identifies areas where motion of water molecules is restricted due to cancer tissue, and the third is dynamic contrast enhanced imaging (DCE) which uses gadolinium-based contrast to find areas where new blood vessels are growing (cancer cells make their own blood vessels to supply themselves with oxygen and nutrients). All of these images are sent to a computer workstation to aid the radiologist in interpreting the images with the goal of finding tumor-suspicious regions. This is called Computer Aided Detection (CAD).
MRI-Targeted Biopsy
A biopsy, or needle aspiration, is a minimally invasive way for doctors to remove small samples of the prostate to identify if the prostate tissue has indeed cancer or not.
At HALO Diagnostics, we use MRI guided biopsy to verify if or what type of cancer is present. MRI-guided biopsy is performed under an MRI that allows the team to guide the biopsy needle in real-time to target exactly the area of the prostate that looked suspicious under the mpMRI, and can ensure a more accurate sampling without damaging healthy prostate tissue.
In comparison, traditional prostate biopsy approaches like TRUS (transrectal ultrasonography–guided biopsy), take a “poke and hope” approach, where biopsy cores are taken with much less clear views on the prostate. TRUS biopsy can miss 30% or more of cancers leading to false negative diagnoses.
Another type of frequently performed prostate biopsy is called an MRI Ultrasound Fusion Guided Biopsy. This approach is more accurate than the traditional TRUS approach and is often performed by a urologist using previously obtained prostate MRI images combined (hence the term fusion) with real-time ultrasound imaging. While sometimes not as accurate as MRI-guided biopsy, MRI ultrasound fusion biopsy is an improvement to the traditional TRUS approach.

Advantages of MRI Guided Biopsy
- Specific targeting of tumor suspicious region instead of random sampling (TRUS biopsy can miss aggressive cancer lesions about 30-35% of the time)
- Minimally invasive with fewer samples taken (3-4) instead of 12 samples with TRUS
- Reduced pain and damage to the rectal wall
- Reduced chances of infection
- Increased sensitivity of detecting aggressive lesions with decreased potential for detecting indolent disease. This reduces overtreatment and prevents the psychological burden with misclassification of cancer stage
- Better spatial localization with specialized software to reach tumor suspicious regions otherwise difficult to reach with TRUS
- TRUS biopsy can underscore lesions due to its inherent random nature. An aggressive cancer could be “skimmed” or completely missed. A low grade tumor might be sampled while an aggressive one is completely missed.
The HALO Solution
At HALO Dx we use Laser Focal Therapy (laser ablation) for the treatment of prostate cancer. This procedure involves placing the patient inside the MRI scanner and displaying MRI images and thermal maps on a computer screen. A thin laser fiber is guided to the tumor and laser energy is applied to kill the cancer cells. The temperature and the extent of the ablation zone are constantly monitored with MRI imaging, allowing HALO Dx to identify if all visible tumors have been destroyed and monitor that no important structures are damaged.
Laser Focal Therapy - Invented by HALO!
In 2010, the team of Bernadette Greenwood, Chief Research Officer at Halo Diagnostics, and Roger McNichols, PhD, developed the system to perform Laser Focal Therapy. The first case in the world was performed by the HALO Diagnostics team of Dr. John Feller, Ms. Greenwood, and Dr. McNichols in 2010.
HALO Diagnostics released interim 10-year results from its Phase II 20-year clinical trial for prostate laser focal therapy in patients with localized prostate cancer. Over 170 men, 45-years or older diagnosed with low-to-intermediate risk or recurrent prostate cancer, volunteered to participate in the study. The results are impressive: less than 1% infection, less than 1% erectile dysfunction and less than 1% incontinence – compared to conventional whole gland prostatectomy which has a risk of up to 50% erectile dysfunction and 25% urinary incontinence. These results, along with a 100% prostate cancer-specific survival rate, are an encouraging development for men looking for a prostate cancer therapy option with a lower risk of side effects.
HALO Dx PROSTATE CANCER CLINICAL TRIAL
WHAT IS A CLINICAL STUDY?
A clinical study is a type of research study. Study staff will explain the clinical trial to you. Clinical trials include only people who choose to take part. If you have any questions, you can ask study staff for more explanation or visit: www.clinicaltrials.gov
WHAT IS THE PROCEDURE?
The laser focal therapy procedure (also called LITT or FLA) is designed to destroy prostate tissue under Magnetic Resonance Imaging (MRI) guidance using laser energy. The laser device is FDA 510(k) cleared for clinical use for soft tissue necrotization. The planning system is FDA 510(k) cleared for MRI guided prostate biopsy. DMI is the first site in the world to combine the devices under IRB approved clinical trial NCT 02243033.
HOW OFTEN WILL I BE SEEN?
After the procedure, you will have an ultrasound of your bladder after 48 hours. After that your follow-up visits will be at 6 months and 1 year. PSA testing and surveys will be done at 3 months, 2 years, 3 years and annually for 20 years. This protocol is to track and study rates of biochemical recurrence and metastasis.
ARE THERE ANY RISKS?
As with many procedures, there are some risks associated with laser focal therapy which can include: pain, bleeding, infection, decrease in seminal fluid volume, incontinence and erectile dysfunction. Study staff will review all the risks associated with the procedure with you and address any questions or concerns.
WHO CAN JOIN THIS STUDY?
Schedule a Consultation
Submit this form and we will contact you, usually within 24 hours, to answer your Prostate screening, diagnosis, and treatment questions.